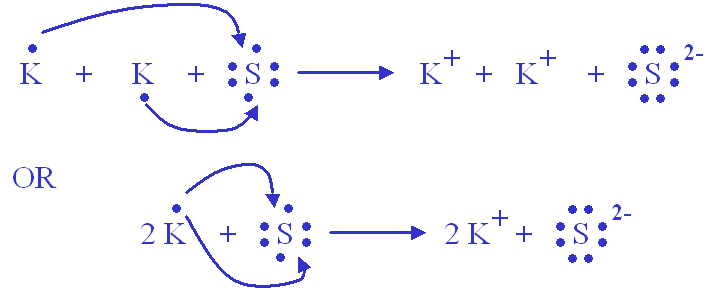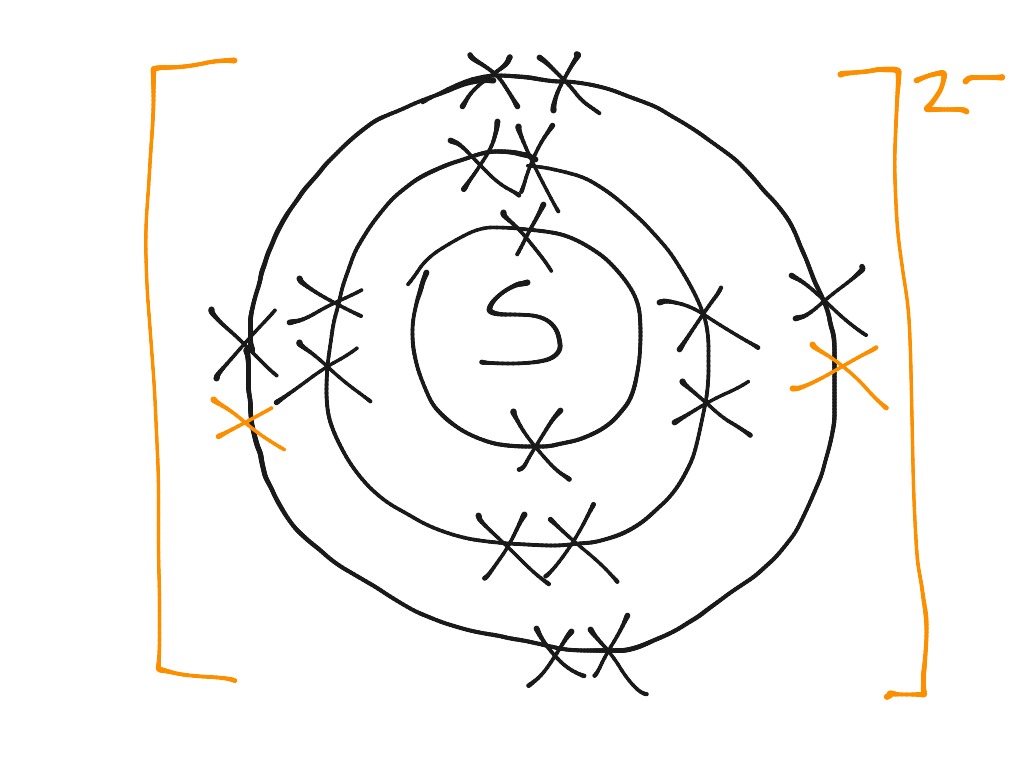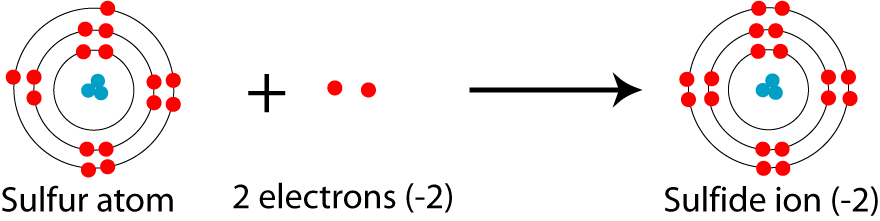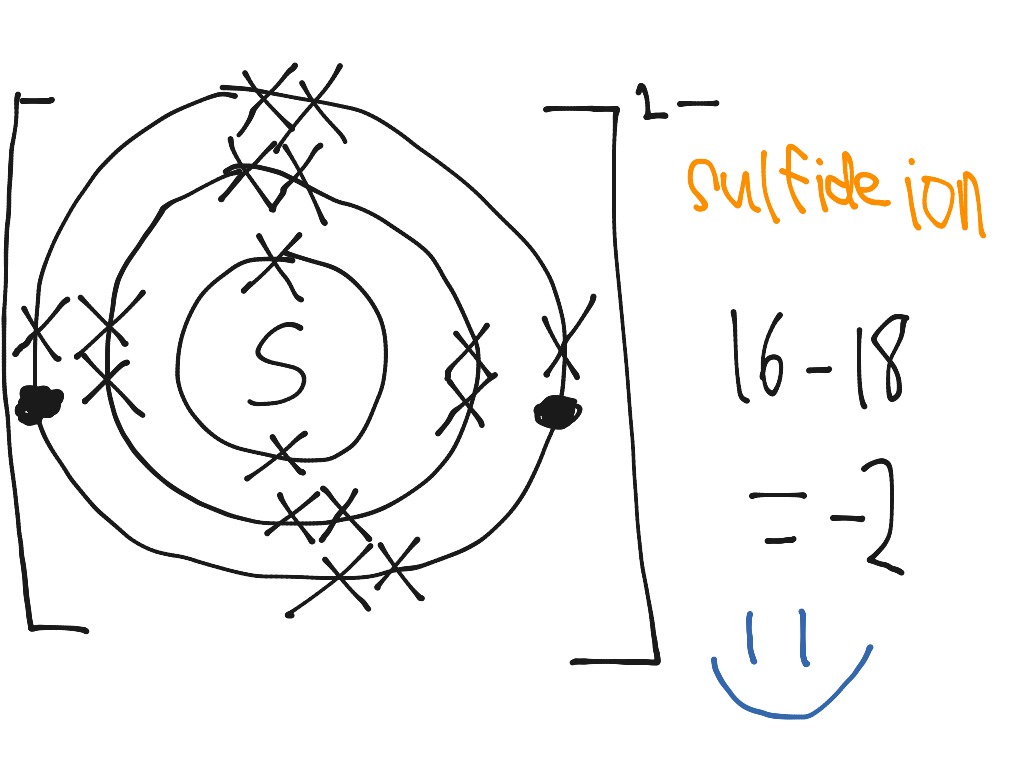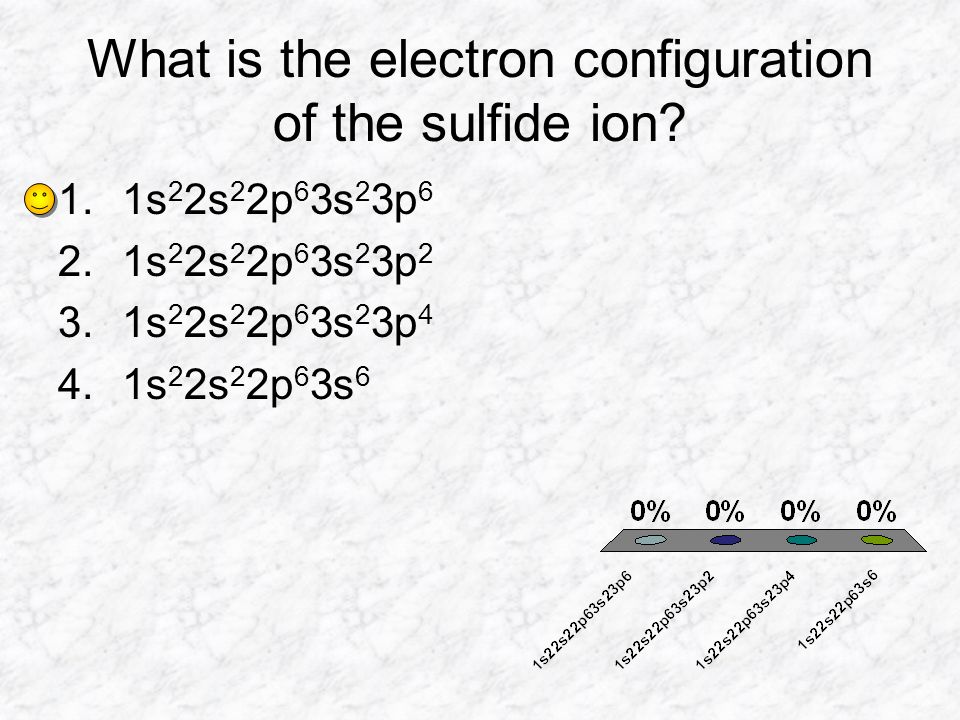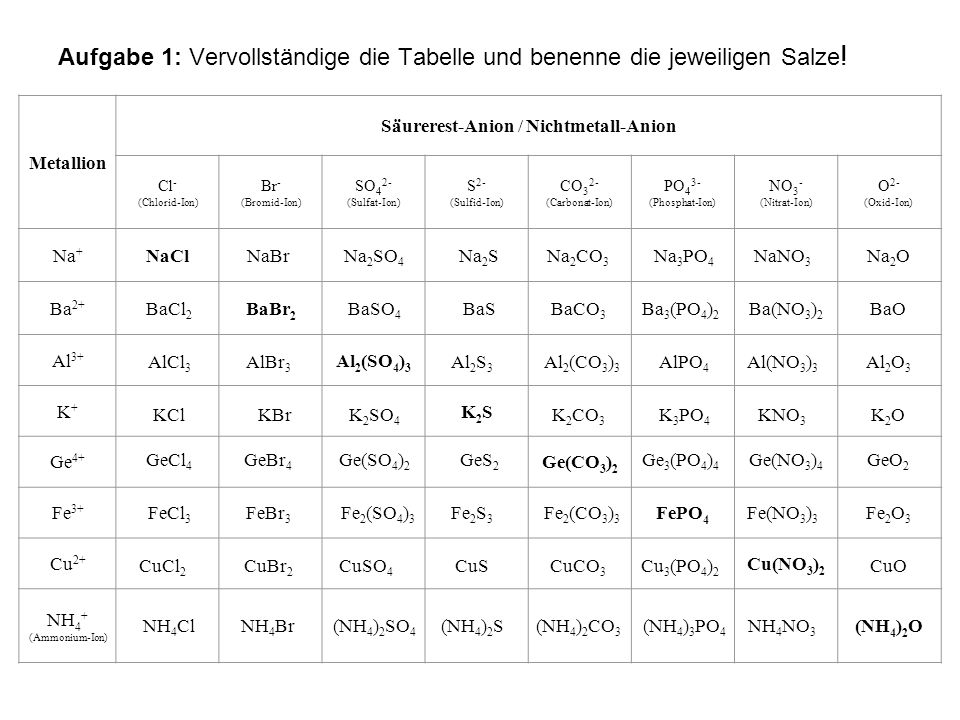
Formeln von Salzen Beim Aufstellen der Formel von Salzen ist darauf zu achten, dass sich die Summe der positiven und negativen Ladungen der kombinierten. - ppt video online herunterladen

SOLVED: A. A sulfide ion (52-) has charge of 2e and is at the origin; where it experiences an electric force of ( 5 x 10-21 , X10-21, 0) N, due to